The shape and structure of a cell are fundamental to its biological function, reflecting the design principle of “form follows function,” widely recognized in architecture and modern design. Translating this principle to artificial cells has been a significant challenge in synthetic biology.
However, breakthroughs in DNA nanotechnology are now providing innovative solutions, enabling the creation of advanced transport channels capable of facilitating the movement of therapeutic proteins across cell membranes.
In this cutting-edge field, Prof. Laura Na Liu, Director of the 2nd Physics Institute at the University of Stuttgart and Fellow at the Max Planck Institute for Solid State Research (MPI-FKF), has developed a groundbreaking tool to control the shape and permeability of lipid membranes in synthetic cells.
These membranes, composed of lipid bilayers enclosing an aqueous compartment, serve as simplified models of biological membranes. They are invaluable for studying membrane dynamics, protein interactions, and lipid behavior. The findings of this research have been published in Nature Materials.
A Breakthrough in DNA Nanotechnology
This innovative tool represents a significant step toward creating functional synthetic cells and has the potential to revolutionize the development of new therapies. Prof. Liu and her team have successfully utilized signal-dependent DNA nanorobots to enable programmable interactions with synthetic cells.
“This work marks a milestone in applying DNA nanotechnology to regulate cell behavior,” says Liu.
The team focused on giant unilamellar vesicles (GUVs), which are simple, cell-sized structures that mimic living cells. By employing DNA nanorobots, the researchers were able to manipulate the shape and functionality of these synthetic cells, opening new possibilities for synthetic biology.
Creating Transport Channels for Proteins and Enzymes
DNA nanotechnology is a core focus of Prof. Liu’s research. She specializes in DNA origami structures—DNA strands folded using shorter, specifically designed DNA sequences called staples.
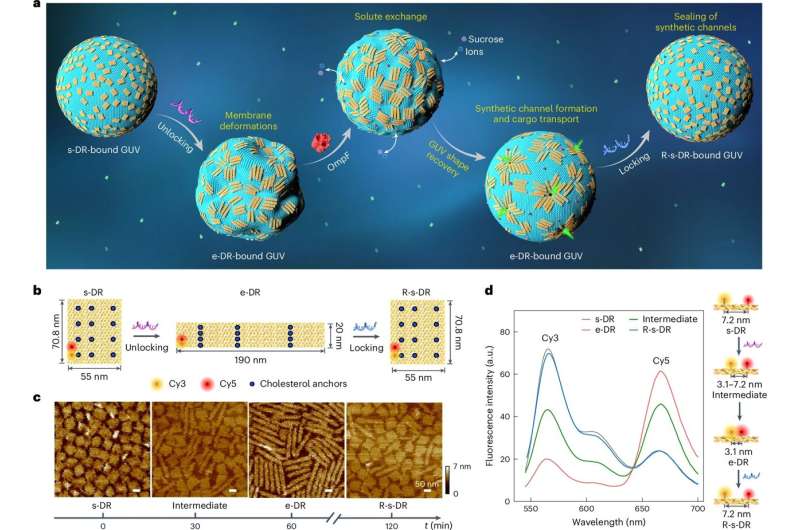
In this study, the team used DNA origami structures as reconfigurable nanorobots capable of reversibly changing their shape, thereby influencing their microenvironment on a micrometer scale.
The researchers discovered that the transformation of these DNA nanorobots could be linked to the deformation of GUVs and the formation of synthetic channels in their membranes. These channels allowed large molecules to pass through the membrane and could be resealed as needed, offering precise control over molecular transport.
Artificial DNA Structures for Biological Applications
Prof. Stephan Nussberger, a co-author of the study, explains, “We can now use DNA nanorobots to design the shape and configuration of GUVs, enabling the creation of transport channels in their membranes. What’s particularly exciting is that the functional mechanism of these DNA nanorobots on GUVs has no direct biological equivalent in living cells.”
This raises intriguing questions: Can synthetic platforms, such as DNA nanorobots, be designed with less complexity than their biological counterparts while still functioning effectively in biological environments?
Advancing Disease Understanding and Therapeutic Development
The study represents a significant step forward in this direction. The cross-membrane channels created by DNA nanorobots allow efficient transport of specific molecules and substances into cells. Importantly, these channels are large enough to accommodate therapeutic proteins and can be programmed to close when necessary.
When applied to living cells, this system could facilitate the targeted delivery of therapeutic proteins or enzymes, offering new possibilities for drug administration and other therapeutic interventions.
“Our approach provides new ways to mimic the behavior of living cells, which could be critical for future therapeutic strategies,” says Prof. Hao Yan, another co-author of the study.
This research not only advances our understanding of synthetic biology but also holds promise for developing innovative treatments for a range of diseases, marking a significant leap forward in the field.
More information: Sisi Fan et al, Morphological transformation and formation of membrane channels in synthetic cells through reconfigurable DNA nanotubes, Nature Materials (2025). DOI: 10.1038/s41563-024-02075-9 www.nature.com/articles/s41563-024-02075-9